Over the last few months, there has been a surge in the market demand for thermal scanners. According to a recent report by marketing research firm, Research and Markets, this increase has been driven in large part by the outbreak of COVID-19 and the use of thermal imaging technology for fever detection, particularly in airports overseas.
Private companies are also driving the market for this technology forward. Following criticisms that it wasn’t doing enough to protect its workers from Covid-19 infection, retail giant Amazon has recently begun using thermal imaging in at least six U.S. facilities to detect potential infections among its employees.
Many smaller businesses in the private sector are beginning to follow suit, investing in these systems with the hope that they will make the workplace safer for their employees. Clearly, the market for these devices is heating up fast as businesses scramble to keep their employees and customers safe. But do they work?
Thermal cameras have been on the market for a long time. So, the technology is not new. However, their use for medical monitoring is. Thermal cameras were never designed for this purpose. While many businesses are feeling the pressure to respond to concerns over worker safety, the jury is still out on whether this technology can provide an effective way to help prevent the spread of the new coronavirus, Covid-19.
This post will highlight some of the most important factors and potential issues that businesses should consider before making significant investments in this technology in their response to Covid-19.
How Does Thermal Imaging Work?
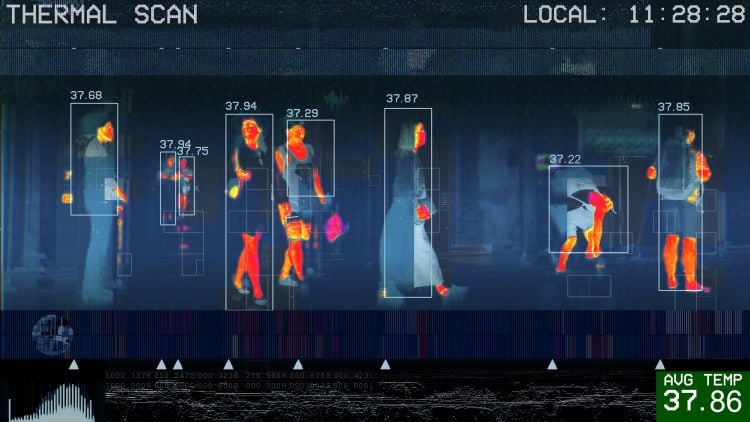
Infrared (I.R.) thermographic cameras measure infrared radiation – a type of radiant energy that is invisible to the human eye but detectable in the form of heat. They use a sensor to measure the amount of infrared radiation (or heat) emitted from an object or in a given area. Measurements are then processed using specialized software to visually display the heat readings in the form of high-definition images or videos. “Hot spots” or areas with higher temperatures will show up as brighter areas on the image against areas with cooler temperatures, which appear darker.
Thermal cameras are considered a “non-contact” device meaning they can be installed in various ways to monitor human body temperature without having to come in contact with the person they are measuring. For example, a thermal camera can be set up in much the same way as the camera where you get your driver’s license. You just step in front of the camera, and it takes a measurement. The technology can also be installed in walk-through scanners such as those used for security in airports. Thermal imaging systems can also be set up like regular security cameras to measure temperatures across a given area. However, in the context of preventing the spread of coronaviruses (or any other type of disease), this type of installation may or may not accurately indicate an individual with an elevated temperature. Rather, the system would identify areas within the space where the temperature exceeds a certain level or range.
Can Thermal Imaging Cameras Detect Potentially Infected People?
According to the most recent information published by the CDC (as of May 31, 2020), people with Covid-19 may not develop a temperature for up to 14 days after infection. In a recent interview with National Public Radio (NPR), Dr. Robert Redfield, director of the Centers for Disease Control and Prevention (CDC), said as many as 25% of people infected with Covid-19 remain asymptomatic. Given these facts and additional studies, some researchers now believe that temperature scanning and similar screening techniques would overlook more than half of those infected.
Thermal cameras pick up the heat emanating from skin, not core body temperature. While the average normal body temperature is generally accepted to be 98.6°F (37°C), what is “normal” can vary by as much as two degrees, from 97°F (36.1°C) to 99°F (37.2°C). Consider, too, that the temperature of a person’s skin is often different from his/her core body temperature and can be affected by environmental factors such as exposure to the sun or sitting in a hot car. Physical exertion and excess body weight can also cause a rise in temperature that has nothing to do with illness.
Therefore, it remains to be seen whether thermal imaging cameras can have any meaningful impact when it comes to preventing the spread of Covid-19 or any other disease.
Are There Any Guidelines or Standards for the Use of Thermal Cameras for Fever Detection?
In April 2020, the U.S. Food and Drug Administration (FDA) issued an enforcement policy for telethermographic systems, which use thermal imaging for measuring body temperature during the Covid-19 pandemic. The policy is intended to support the FDA’s efforts to expand the availability of thermographic systems for use as a triage measure to address the urgent public health concerns related to Covid-19. But, are these devices effective in preventing the spread of the disease?
According to the FDA policy, when “intended for use in the diagnosis of a disease or other conditions or in the cure, mitigation, treatment, or prevention of disease,” such technologies meet the federal definition of a medical device.
The FDA policy does not explicitly state that this technology is effective for the purposes of disease prevention. Instead, it states that its purpose is to “help ensure the availability of products that might offer benefit to healthcare providers and the public during the public health emergency” (emphasis added). The basic requirements of the FDA policy include:
- Devices must be tested to ensure they meet specific performance requirements described in the International Electrotechnical Commission (IEC) Standards or similar standards.
- The devices must be prominently labeled with a notice that provides clear statements of the following:
- Measurements from these devices “should not be solely or primarily relied upon to diagnose or exclude a diagnosis of COVID-19, or any other disease”.
- An elevated body temperature measured by a thermographic device for the purposes of such screening should be confirmed with a secondary evaluation method such as a non-contact infrared thermometer (NCIT) or clinical grade thermometer.
- Public health officials should determine the significance of any fever or elevated temperature measured by these devices.
- The technology should be used to measure the temperature of only one person at a time.
- Visible thermal patterns (e.g., the thermal patterns produced by a group of people gathered in one place) should only be used to determine smaller groups of people within the area of elevated temperature to measure.
- The labeling should also provide the following information:
- The device’s performance characteristics, including its accuracy and the methods and frequency of calibration.
- How to use the device to ensure a measurement within the stated accuracy.
- A description of how the device compensates for thermal drift and the importance of this compensation to obtaining accurate results.
- The part of a person’s body that will be measured.
- How different environmental and system setup factors might affect the measurements (e.g., the screening background, ambient temperature, humidity, airflow, etc.).
- Different factors that should be considered in the design of the facility protocol, such as the installation of the devices, their viewing angle, and the blackbody temperature reference source.
- The procedures for installation and qualification testing that should be performed during installation.
The labeling must also highlight the differences in design, indications, or functions as compared to the unmodified, FDA-cleared version of the product to help ensure that people are aware that it is being used for a purpose other than that for which it was originally approved. Alternatively – and perhaps more clearly – the label can simply indicate that the device is neither FDA-cleared or approved.
Are They Worth the Investment?
Smart business owners evaluate the cost-effectiveness of every significant investment. Aside from the questions remaining about their efficacy and with price tags currently as high as $30,000 per system, there are also other important factors to consider before investing in this technology for the purpose of preventing the spread of Covid-19.
No one really knows how long it might be until a vaccine is developed, and even then, how long that vaccine might remain effective. Eventually, the hope and expectation are that there will be a vaccine widely available for Covid-19. In the meantime, does the cost of installing this technology justify the benefits to your business against what could potentially be a short term risk?
There are also other questions to consider. It’s one thing to purchase and install a technology. But, you also need to consider how you will implement it. For example, who are you planning to monitor? What will your policy be if an employee is detected as potentially infected?
The Department of Homeland Security (DHS) has recently indicated that it is considering using such technologies at airports in the U.S. The airline industry is a heavily regulated industry with policies already in place for dealing with passengers perceived as a potential threat. However, most businesses do not have such procedures in place.
If you are considering monitoring not only your employees but also your customers as they enter your business, there is a whole host of other factors to consider. How will you respond when the system detects a potentially infected person? Will you forbid that customer to enter your business? Will you insist that the customer wear a mask? In either case, stopping customers at the door could be detrimental to your business as that customer is highly likely never to return. Thus, implementing this technology in this way could be significantly harmful to the long term health of your business. Quickly eroding any benefit they might have had.
There are also potential legal questions to consider and which presently appear to have no clear answer. For example, is a person’s body temperature considered private health information, which is protected under the federal Health Insurance Portability and Accountability Act (HIPAA)? If so, using such scanners to screen people coming in and out of the workplace may be a violation of federal law.
Contact Koorsen
Given all these potential issues, the use of thermal cameras for monitoring temperature is something that businesses should consider very carefully before they invest. The fact is that many of these questions surrounding their use for this purpose have yet to be answered because while the technology is not new, their use for medical purposes is.
As a leading company in the design and installation of security systems, including thermal imaging technology, Koorsen is currently evaluating the use of thermographic devices to monitor the temperature of people entering a building.
While we cannot make any specific recommendations regarding their effectiveness and legality for use in your business, our experts can help you evaluate whether any thermographic devices you might be considering for this purpose meet the FDA guidelines. Contact Koorsen today, before you invest in a technology that may not live up to its promises.




